Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Author affiliation: Universidad Internacional SEK, Quito, Ecuador (D. Romero-Alvarez); University of Kansas, Lawrence, Kansas, USA (D. Romero-Alvarez, A.T. Peterson); Universidad de las Américas, Quito (M. Calvopiña, E. Cisneros-Vásquez, C. Bastidas-Caldes, J.H. de Waard, D. Silva-Martinod); Universidad San Francisco de Quito, Quito (D. Garzon-Chavez); Colorado State University, Fort Collins, Colorado, USA (A.K. Warren, L.S. Bennett, R.R. Janapati, M. Jackson, C. Avanzi); Agrocalidad, Quito (M. Cabezas-Moreno); Centre Hospitalier de Cayenne, Cayenne, French Guiana (R. Schaub)
The World Health Organization Global Leprosy Strategy targets the long-term goal of leprosy elimination through interruption of disease transmission (1). One factor that can impair that goal is environmental or animal reservoirs that contribute to persistence of the Mycobacterium leprae bacteria and potential spillover into the human population. The Strategy acknowledges that M. leprae zoonotic transmission exists but with a lower risk and highly localized in North America (1), possibly because of a lack of research on new and existing animal reservoirs in other locations.
M. leprae, the main causative agent of leprosy, has a broad range of animal hosts, including wild armadillos (Dasypus spp.) in the Americas, red squirrels (Sciurus vulgaris) in the British Isles. and nonhuman primates in the Philippines and Africa (2). Armadillos are a family of medium-sized mammals, belonging to the Xenarthrans clade, which also includes sloths and anteaters (3). At least 20 armadillo species have been recognized (3). The Cingulata order encompasses >9 Dasypus species, including the nine-banded armadillo (D. novemcinctus), considered the main M. leprae reservoir in the Americas (2).
Ecuador, located in northwestern South America, is a medium-income economy nation with ≈18 million inhabitants (4). Officially, Ecuador eliminated leprosy as a public health threat, which means incidence is <1 new case/10,000 inhabitants; only 41 new leprosy cases were registered in 2022 (5). Scientific literature on leprosy in Ecuador is scarce; nonetheless, the Ministry of Public Health suggests a higher disease incidence across the country (6). Armadillos are found throughout Ecuador and are valued as a protein source and a cultural item in many rural settings (7). In view of the uncertain epidemiologic landscape of leprosy in Ecuador and the occurrence of a possible animal reservoir in the country, we investigated M. leprae infection in armadillos in Ecuador.
We gathered tissue samples from 45 armadillos via local hunters who use the mammal as a protein source for their families and communities. The Instituto Nacional de Biodiversidad (Quito, Ecuador) also donated 3 additional samples stored in 70% ethanol, for a total of 48 armadillos. We performed tissue collection according to a protocol approved by the Ministerio del Ambiente, Agua y Transición Ecológica, as part of the Genetic Resources Access Framework contract (contract no. MAATE-DBI-CM-2021-0172). We established definitive armadillo species identification by morphological features, known geographic distributions, and molecular diagnosis (Appendix 1). We processed >2 tissues from 36 armadillos and only 1 tissue for the other 12; we examined each tissue >2 times (Appendix 1). We performed DNA extraction and pathogen identification via real-time quantitative PCR (qPCR) using previously well-established primers and protocols (8,9) (Appendix 1). We considered a sample positive for M. leprae or M. lepromatosis only if 2 independent qPCR runs yielded a cycle threshold (Ct) <35 (9,10).
We processed a total of 84 armadillo tissue samples (Appendix 2), including 38 (45.24%) liver, 26 (30.95%) spleen, and 10 (11.9%) muscle samples (Table). We identified M. leprae DNA in 13 (15.48%) samples, mostly from liver (n = 8/38 [21.05%]) and spleen (n = 4/26 [15.38%]) (Table). For 3 armadillos with varying results between tissues, the liver was the source of positivity (Appendix 2). All 84 tissue samples were negative for M. lepromatosis according to our protocols.
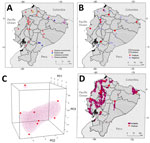
The 48 individual armadillos belonged to 4 different species: 40 (83.33%) were D. novemcinctus, 6 (12.5%) Dasypus spp. (not identified to species), 1 (2.08%) D. pastasae, and 1 (2.08%) Cabassous centralis (Table; Figure). We detected M. leprae in 9 D. novemcinctus armadillos, for an overall prevalence of 18.75%. Ct values were 26.01–33.66 (Table, Figure; Appendix 2). Most (20.83%, 10/48) armadillos were collected in the Esmeraldas province along the coast, among which only 1 (10%) D. novemcinctus armadillo was M. leprae–positive (Figure, Table). We observed the highest prevalence (42.86%) of infected armadillos in Santo Domingo de los Tsáchilas, in the northwest, where 3 of 7 animals were M. leprae–positive (Figure).
To characterize potential clusters of infected D. novemcinctus armadillos in Ecuador, we used the localities of the 9 M. leprae–positive armadillos to develop a species distribution model based on 1-class support vector machine hypervolumes (11) and 20 environmental predictors (Figure, panel C; Appendix 1 Figure 1). The subtropical region of Ecuador, west of the Andes mountains, had the highest concentration of environments like those with M. leprae–positive detections (Figure, panel D). Specifically, Esmeraldas, Los Ríos, Santo Domingo de los Tsáchilas, Santa Elena, northern Bolívar and Guayas, and southern Manabí are regions with environmental similarities to locales were infected D. novemcinctus armadillo were found (Figure).
The canon of leprosy transmission has been actively rewritten in the past 2 decades (2). Confirmation of zoonotic M. leprae transmission in the United States (7) prompted a series of studies to evaluate the spread of leprosy bacilli in the D. novemcinctus armadillo across its range in the Americas (Appendix 1 Figure 2). Our research demonstrated that nine-banded armadillos from the 3 continental regions of Ecuador host M. leprae with an 18.75% prevalence (Table; Figure). Detection of bacilli in wild armadillos is the first step in evaluating leprosy as a zoonotic pathogen in Ecuador. All 84 tissues examined were negative for M. lepromatosis, in agreement with previous results for other mammals in Europe and Mexico (12,13) and for armadillo specimens from across the Americas (8).
One limitation of our study was that our sampling scheme depended on local hunters who collect armadillos; thus, systematic sampling representing specific ecologic regions was unfeasible. Moreover, sampling scope was initially limited to tissues in ethanol, preventing serology and histopathology investigations. Nevertheless, we were able to collect tissues from across the country and observed consistency in the molecular detection of M. leprae with multiple rounds of qPCR in DNA extracted from various tissues from the same armadillo (Table; Appendix 2). Of note, a Ct value <35 in 2 independent qPCR rounds per tissue as criteria for M. leprae positivity is conservative, yet informative of the pathogen in nine-banded armadillos in the country.
Ecuador hosts at least 5 armadillo species: Cabassous centralis, C. unicinctus, Priodontes maximus, D. pastasae, and D. novemcintus (14). D. novemcintus was the most common (83.33%) armadillo species in our sampling and the only M. leprae–positive species (Table). We identified 3 other armadillo species, but all were M. leprae–negative (Figure, Table). Apart from D. novemcinctus, armadillo species in which M. leprae has been identified beyond Ecuador include Euphractus sexcinctus, Dasypus spp. nov., and D. sabanicola. Moreover, D. septemcinctus armadillos have been shown to be susceptible to M. leprae laboratory infections (15).
Species distribution models have seldom been used to characterize leprosy geographic range. Given the epidemiologic tenets of M. leprae, including long incubation period and human-to-human transmission, data for those models is difficult to obtain. Moreover, information on M. leprae prevalence in armadillos is either overrepresented as in the southern United States, or scant and dispersed as in the rest of the Americas (2) (Appendix 1 Figure 4). Thus, by leveraging our M. leprae–positive armadillo detections across the landscape of Ecuador, our model depicted clusters of environmental similarity. Considering the inherent challenges to collecting and studying armadillos (3), our model could be used to optimize future expedition sampling.
In conclusion, presence of a nonhuman M. leprae host carrier, the nine-banded armadillo, is likely to contribute directly or indirectly to the human leprosy incidence in Ecuador and other countries and will likely impair long-term goals of disease elimination. However, the detection of M. leprae in armadillos from Ecuador should exemplify how continued sampling and surveillance in wildlife can avert future zoonotic infections.
Dr. Romero-Alvarez is an infectious disease eco-epidemiologist from Ecuador working as an associate professor in the Research Group of Emerging and Neglected Diseases, Ecoepidemiology and Biodiversity, in the Faculty of Health Science at the Universidad Internacional SEK (UISEK), Quito, Ecuador. His research interests include ecology of infectious diseases, zoonotic surveillance, and spatial epidemiology.