Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Author affiliation: Hospital Militar Central, Bogotá, Colombia (L.J. Medina-Lozano, S.A. Bolívar Lozano, C. Guavita, D.C. Gutiérrez-González, Á.A. Faccini-Martínez); Universidad Militar Nueva Granada, Bogotá (S.A. Bolívar Lozano, C. Guavita, Á.A. Faccini-Martínez); Universidad del Rosario, Bogotá (M. Camargo, L.H. Patiño, J.D. Ramírez); Icahn School of Medicine at Mount Sinai, New York, New York, USA (J.D. Ramírez)
Malaria is the most common life-threatening tropical disease associated with fever among returned travelers from sub-Saharan Africa. During 2010–2013, according to the World Malaria Report 2023, the Comoros Islands reported a total of 144,546 cases of Plasmodium falciparum infection and 1,571 cases of P. vivax infection (1). Nevertheless, during 2014–2022, only P. falciparum cases were reported, without P. vivax cases or mixed infections (1).
Data collected by the GeoSentinel Surveillance Network for 1,415 ill travelers returning from Indian Ocean islands during 1997–2010 indicated that the proportion of mosquitoborne infections (including malaria) was higher among travelers to the Comoros than among other travelers (2). At the same time, studies published in the past 10 years reported malaria cases exported from the Comoros to other countries during 1999–2021, mainly to territories of France (France, Réunion, and Mayotte) and 1 case to Japan; the most common etiologic agent was P. falciparum (≈255 cases), followed by P. ovale (≈19 cases) and P. vivax (≈11 cases) (3–7). We report a case of fatal mixed Plasmodium malaria infection in a man who returned to Colombia from the Comoros in 2024.i
On June 14, 2024, an otherwise healthy 50-year-old male former military service member sought care at a primary care center in Bogotá (capital city of Colombia) after 7 days of fever (up to 39°C), chills, diaphoresis, myalgias, arthralgias, and headache. He reported a 2-day history of epigastric pain, loose stools, and dark urine. His illness was considered an unspecific viral infection, and he was discharged. His signs/symptoms had begun 10 days after he returned from Grande Comoro Island, where he had stayed for 2 weeks while providing military training. Until his travel to the Comoros, he had not been in another P. vivax/P. falciparum–endemic area in the previous 5 years. On June 15, 2024, he was admitted to Hospital Militar Central, a reference military hospital in Bogotá, for a syncopal episode, disorientation, and jaundice. Physical examination revealed hypothermia, tachycardia with Kussmaul breathing, and reduced oxygen saturation. The patient was jaundiced and stuporous with no bleeding.
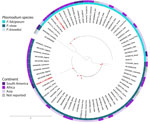
Laboratory tests revealed leukocytosis, anemia, severe thrombocytopenia, malarial hepatopathy, renal impairment, metabolic acidosis, and hyperlactatemia (Table). Thick and thin blood smears showed P. falciparum (17,840 trophozoites/μL; parasitemia of 0.35%) with gametocytes and P. vivax (8,320 trophozoites/μL). Severe malaria was diagnosed, and treatment with intravenous artesunate was initiated (2.4 mg/kg) in addition to fluid resuscitation and invasive mechanical ventilation support. However, the patient experienced 2 episodes of cardiopulmonary arrest and died. Autopsy and histopathologic examination of heart and brain samples revealed multiple parasitic structures compatible with Plasmodium trophozoites (Appendix Figures 1, 2). PCR performed on blood smears confirmed the presence of P. falciparum and P. vivax (Appendix). DNA gene fragments from the small subunit rRNA 18S gene were sequenced from the positive specimens, and phylogenetic analyses positioned the obtained sequences in the same subclade as P. falciparum sequences detected in South Africa and as P. vivax sequences detected in Cameroon, Nigeria, China, and India (Figure; Appendix). Sequences were deposited in GenBank (P. falciparum accession no. PQ408861, P. vivax accession no. PQ408862).
In the most recent study that used PCR to assess the distribution of Plasmodium spp. on Grande Comoro Island, among 159 positive samples collected during 2012–2013, nearly all (98.11%) were positive for P. falciparum and only 1.25% were positive for P. vivax (8). At that time, the authors indicated that routinely, without PCR testing, the rapid diagnostic tests used in the Comoros were able to identify P. falciparum but no other Plasmodium spp. (8), which is in accordance with a recent published editorial that discusses the contemporary concern with regard to the need to re-evaluate the spread of P. vivax in sub-Saharan Africa (9). The editorial mentioned that during 2017–2021, among 1.57 billion malaria rapid diagnostic tests purchased for use by sub-Saharan Africa national malaria control programs, 79.4% were focused on identifying P. falciparum and the remainder were combination tests lacking P. vivax specificity; thus, the predominant approach for malaria diagnosis across Africa was unable to specifically detect P. vivax (9). Our report highlights the value of strengthening laboratory diagnostic tools with good performance for detecting and accurately identifying Plasmodium spp. in clinical settings and of conducting more genetic-epidemiologic studies in the Comoros and other sub-Saharan Africa countries.
Dr. Medina-Lozano is an internal medicine physician at Hospital Militar Central in Bogotá, Colombia. His research interests primarily focus on tropical infectious diseases.